Abstract
High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is considered the standard of care as first-line therapy for eligible multiple myeloma patients(Barlogie et al. 1987; McElwain & Powles 1983). Median age at diagnosis of multiple myeloma is 69 years old (SEER 18 2010-2014). Data from the CIBMTR revealed that the number of transplants done per year and the age of recipients are still rising(D'Souza & Zhu 2016). With an expected myeloma prevalence rising by 57% from 2010 to 2030(Smith et al. 2009), the number of older patients considered for AHSCT will increase significantly. Best treatment options for those older patients must therefore be addressed. The assessment of hematopoietic cell transplant comorbidity index score (HCT-CI) (Sorror et al. 2005; Sorror et al. 2009) can be helpful in the selection of candidates for AHSCT. The goal of this study was to identify factors impacting the safety and efficacy of AHCT in older multiple myeloma patients in order to better select those who will benefit from such an intervention.
This single center, retrospective study examines outcomes of AHSCT in elderly patients (≥60 years old) with multiple myeloma compared to younger patients (<60 years old). Between January 1, 2011, and September 1, 2015, 93 patients met the inclusion criteria and were included in the study. Patients signed an informed consent and the ethics committee of our institution approved the study. Toxicity was analyzed according to age and HCT-CI. Progression-free-survival (PFS) and overall survival (OS) were analyzed according to age at the time of transplantation, HCT-CI, Revised International Staging System (R-ISS) and disease status at the time of transplant.
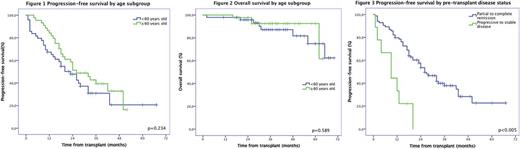
From the 93 patients included in the study, 49(53%) were included in the younger patient group with a median age at transplant of 54 years-old (range 34 to 59). Forty-four (44) patients were included in the older patient group with a median age at transplant of 65 years-old (range 60 to70). Two thirds (66%) of patients were male. Based on the R-ISSS score, 46% were stage I, 54% were stage II and none were stage III. HCT-CI score was low, intermediate and high in 40%, 32% and 28% of patients respectively. Median karnofsky index was 90 (range 70 to 100). Acute renal failure was more frequent in the older group (p=0,028) at multiple myeloma diagnostic. Light chain disease was more frequent in the younger patient group with 19% compared to 3% (p=0,09). Mucositis was similar in occurrence in both groups with only 5% of grade ≥3 mucositis. No parenteral alimentation was required in the older patient group while 4 patients required it in the younger patient group(p=0,053). The incidence of febrile neutropenia was universal in all patients (100%) within the cohort. The incidence of septic shock and admission to intensive care unit was only 1%. Delirium was diagnosed in 2 older patients (p=0,131). There was no difference in toxicity profile when comparing both groups. Median engraftment for neutrophils was 14 days and 16 days for platelets. Median hospital stay duration was 21 days. Intravenous antibiotics were used a median time of 8 days. There was no difference in resources used during transplant in both groups. Median CD4 count was comparable in both groups at 3 months and at 1 year after transplant. With a median follow-up of 37 months (range 2 to 70), our cohort had a PFS of 24 months with no difference between younger and older patient groups(p=0,234). The median OS was not reached at the time of evaluation for both groups. Estimated 4 years PFS was 21% and OS was 81%. There was no difference between our younger and older patient cohorts (figure 1 and 2). Being in stable or progressive disease was associated with worse PFS (median of 9 months versus 26 months, p<0,0005)(figure 3). Our 1 year NRM was 0% in both groups.
In conclusion, AHSCT is a good treatment option in well-selected patients. Comorbidities must be considered and HCT-CI can help clinicians choose carefully those patients. Our study demonstrates that age should not be the limiting factor when considering ASCT in older patients. Toxicity and survival were comparable in both groups. Best timing when to proceed to ASCT is still in investigation. Considering the significantly worse PFS with chemoresistant disease, other treatment options should be considered before AHSCT without age being a consideration.
Delage: AbbVie: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal